Epigenetic Imprints of Immune Memory across the Human Body
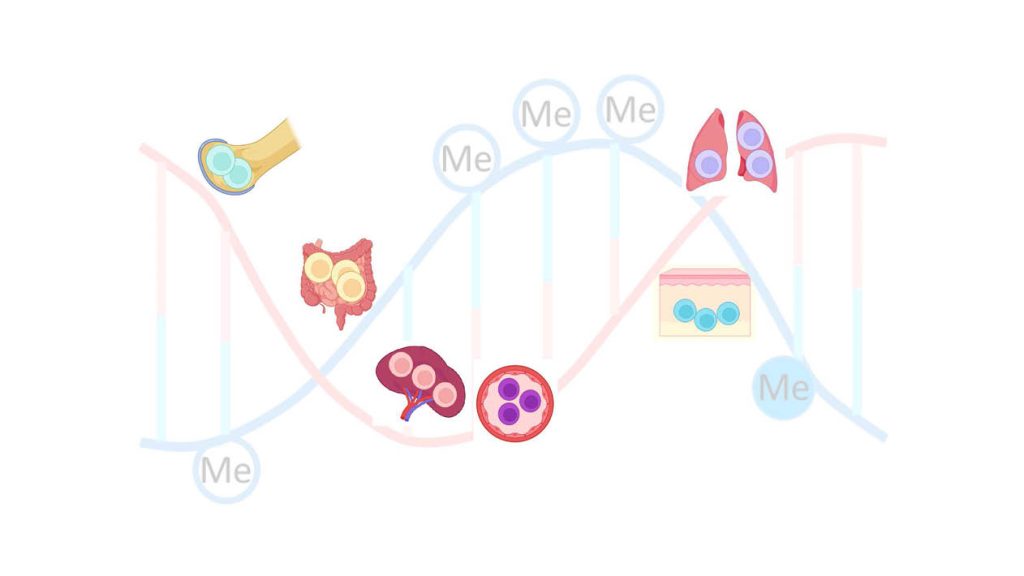
Epigenetic programming of immune cells: permanent tissue residence and protection against infections
A remarkable collaborative study by the research teams of Andreas Radbruch at the German Rheumatology Research Centre Berlin, an institute of the Leibniz Association, and Jörn Walter at the University of the Saarland, together with scientists from the Charité University Medicine and Berlin Institute of Health, has discovered how immune cells are epigenetically imprinted to provide protection against repeated infections by pathogens and to persist in tissues. The findings are published in the journal Immunity & Inflammation recently.
T lymphocytes are an essential component of this “Immunological Memory”. This study shows that “memory T lymphocytes” modify the genes they need to survive and persist in specific tissues, as well as the genes required to mount protective responses. The mechanism involves epigenetic imprinting through demethylation of particular cytosine bases, which flags these genes for transcription factors. While this process optimizes immunity against pathogens, it can also become misdirected, contributing to diseases, such as allergies and chronic inflammation.
The authors present the first comprehensive atlas of genome-wide demethylation patterns in memory T lymphocytes across human bone marrow, intestine, spleen, lung, skin, and blood. The findings show that memory T lymphocytes are differentially imprinted for residency and survival in each individual tissue. This atlas provides a valuable resource to identify molecular pathways critical for long-term immune maintenance and to explore novel options for treatment of chronic immune-mediated diseases.
According to Dr. Jun Dong from the German Rheumatology Research Centre Berlin, corresponding author of the study: “Our results reveal that T lymphocytes are not only imprinted for recall responses but also for residency in particular tissues. Their epigenetic imprint offers a unique opportunity to track them during secondary and chronic immune responses. We are gaining new insights into the division of labor in immunological memory—both in protective immunity and in immune-mediated diseases such as rheumatic disorders.”
The study was supported by the German Research Foundation (DFG), the Leibniz Collaborative Excellence Programme, the China Scholarship Council (CSC), the Chinesisch-Deutsches Zentrum für Wissenschaftsförderung (CDZ, DFG), the European Research Council (ERC), and the German Epigenome Programme ‘DEEP’.
Original publication
Cell Biology

Acknowlegements
The study was supported by the German Research Foundation (DFG), the Leibniz Collaborative Excellence Programme, the China Scholarship Council (CSC), the Chinesisch-Deutsches Zentrum für Wissenschaftsförderung (CDZ, DFG), the European Research Council (ERC), and the German Epigenome Programme ‘DEEP’.
Tissue illustrations was generated using BioRender.











