Revisiting Tissue Residency: CD69⁻ Memory T Cells in the Human Bone Marrow
Retention of Surface CD69-Negative Memory T Cells in Bone Marrow via S1PR1 Internalization
Tissue-resident memory T cells play a central role in inflammatory rheumatic diseases, but little is known about how they are maintained in the inflamed tissue. Emilia Schneider Revueltas and colleagues from the group of Prof. Dr. Andreas Radbruch at the German Rheumatology Research Center, a Leibniz Institute, now compared the gene expression of memory T cells circulating in the blood and and those residing inside the bones, a major reservoir of memory T cells. They discovered that some of the cells do not express the chemokine receptor required to exit into the blood. Other cells do express this receptor, but internalizes it, together with a protein called CD69. These surface CD69-negative memory T cells of the bones express different genes than those in the blood, supporting the notion that they are tissue-resident. The study was recently published in the European Journal of Immunology.
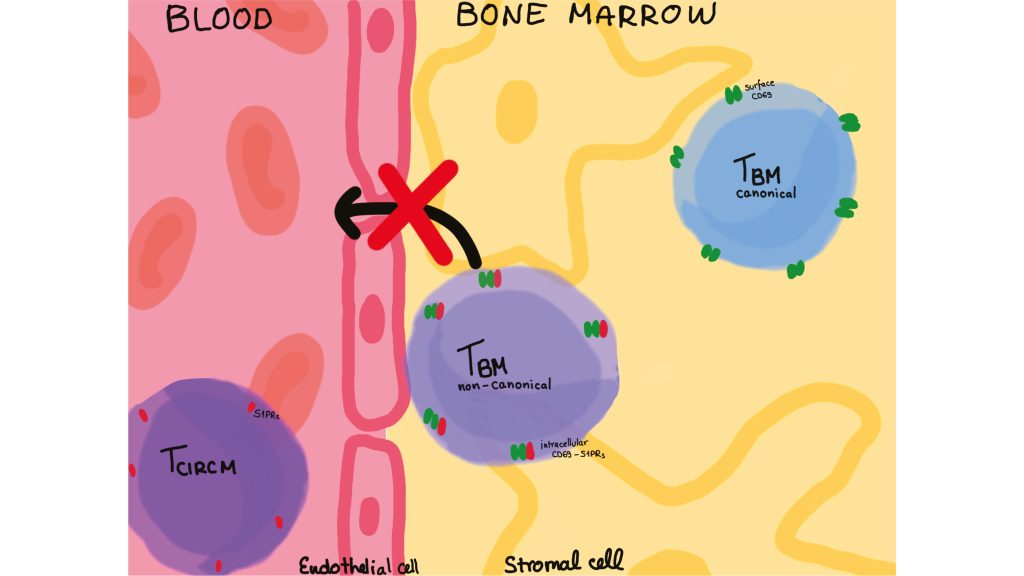
Tissue-resident memory T cells are defined by their expression of the surface marker CD69 and the lack of sphingosine-1-phosphate receptor 1 (S1PR1), a chemokine receptor which senses sphingosin-1-phophate in the blood and promotes exit of cells from tissues into the blood. In a recent publication, researchers at the German Rheumatology Research Center (DRFZ) compared single-cell transcriptomes and T cell receptor (TCR) repertoires of memory T cells from the blood and bone marrow of human donors.
Emilia Schneider Revueltas and colleagues found that memory T cells from blood and bone marrow show similar, but distinct gene expression profiles, but have different repertoires of antigen receptors. This identifies memory T cells of the bone marrow as a distinct, tissue-resident compartment of memory T cells.
A minority of bone marrow-resident memory T cells expresses the marker CD69, but not the S1PR1 chemokine receptor, and thus is not attracted to the blood. The majority does not express CD69 on the cell surface and it had been unclear whether they are true residents of the bones. The DRFZ researchers found that these cells do express CD69, and also S1PR1, but both molecules dimerize and the dimer is internalized by the cells, neither molecule is expressed on the cell surface, so that the cells are also blindfolded for egress into the blood. This mechanism may also contribute to the retention of inflammation-driving T cells in chronically inflamed tissues and presents a potential target to prevent the systemic spread of inflammation.











