Intestinal Microbiota & Immune System
Dialog between the intestinal microflora and the immune system
Billions of bacteria live in the intestine. Usually the intestinal flora (link to glossary) provide vital functions to help us stay healthy. The bacteria in the healthy intestinal flora:
- Help us to digest food and to produce vitamins
- Protect us against the invasion of pathogens
- Strengthen our immune system
- Reduce inflammation in the entire body
Approximately 1 in every 10 people in Germany suffers from a chronic inflammatory disease such as Rheumatoid Arthritis, psoriasis, or intestinal inflammation. In these diseases the intestinal flora is often very different compared to healthy people.
Intestinal flora as a mirror of health
An unbalanced intestinal flora is likely to affect our health, whereas a balanced intestinal flora can even protect against diseases. It can influence the course of the disease and even the response to therapy. During treatment for these chronic inflammatory diseases the intestinal flora can also change. The composition of the intestinal flora in patients affected by chronic inflammatory diseases such as Rheumatoid Arthritis differs from that of healthy people. Often, the diversity of bacteria is reduced in these patients. The fewer different bacteria are present within the intestine, the more susceptible the gut is to inflammation. This reduction in the diversity of intestinal flora has been associated with other conditions such as cancer and depression.
IgA antibodies as a protective barrier for a strong intestinal flora
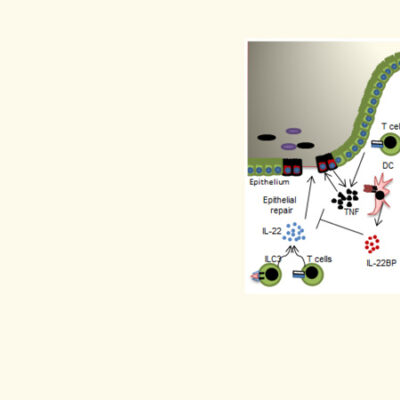
The white blood cells present in our intestines produce IgA antibodies. These antibodies interact with the microbiota in many ways and help to regulate the diversity of the intestinal flora. They form a protective barrier against pathogens. IgA antibodies also bind directly to the bacteria of the intestinal flora and thus prevent them from entering the body via the intestinal mucosa. Our scientists are investigating which bacteria increase IgA formation in the intestine and how they do this.
The DRFZ is particularly interested in the link between these IgA-promoting bacteria and preventing inflammation
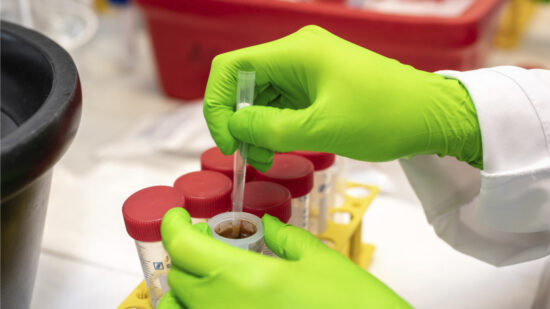
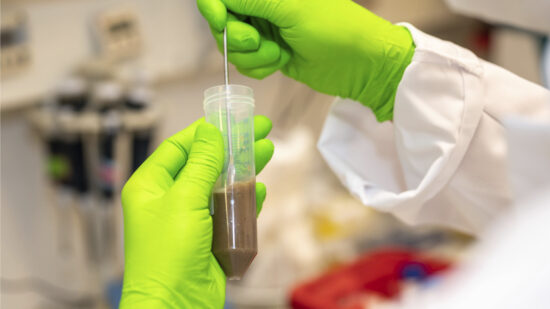
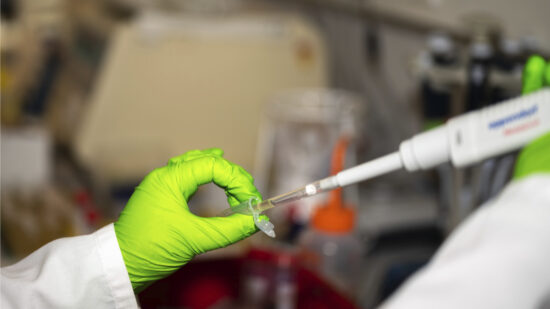
Microbiota cytometry developed to study the intestinal flora
The DRFZ has developed a new method, microbiota flow cytometry. It can analyse the composition of the intestinal flora from stool samples quickly and easily. With this method we compare the intestinal flora of healthy and diseased people.
We are currently testing whether:
- microbiota cytometry can be used for disease diagnosis, and
- whether the composition of the intestinal flora can be used to predict how successful a treatment, e.g. a particular drug, will be.
If this succeeds, diseases could be detected earlier – and treated earlier and more specifically.
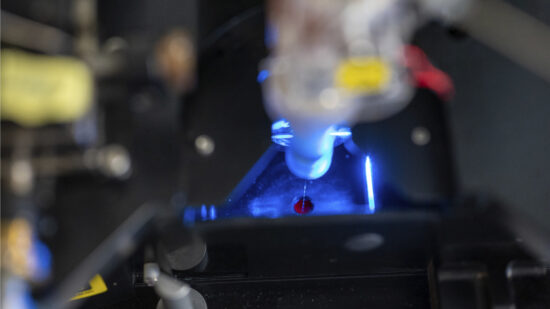
Microbiota Cytometry
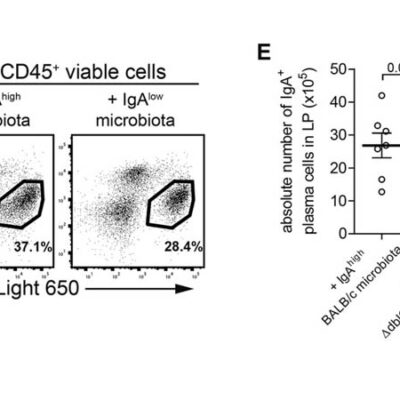
High-resolution microbiota cytometry is a new method for the analysis of the intestinal flora. For the first time, it allows for the fast and easy detection of individual bacterial populations of the intestinal flora based on their DNA content, their light-scattering properties, and their staining with fluorescent antibodies.
Individual bacterial populations can even be sorted and isolated for more detailed investigation. This method is constantly being developed by testing and validating new parameters. With the help of this method, the DRFZ has already easily and successfully detected dramatic differences in bacterial composition:
- in the intestine of mice with intestinal inflammation compared to healthy mice, and
- in patients with intestinal inflammation compared to voluntary donors.
The DRFZ developed the method together with the Gastroenterology Department of Charité – Universitätsmedizin Berlin, Campus Benjamin Franklin and the Helmholtz Centre for Environmental Research in Leipzig.
If you have current medical problems, please consult a doctor. No patients can be treated at the DRFZ. Below you will find links where you can find out where to go as a patient.

 Deutsch
Deutsch